Article
Staying aware 365 days a year: Driving safe care with clinical risk identification and analysis processes
* This content was originally published prior to N. Harris Computer Corporation’s 2022 acquisition of the Allscripts Hospital and Large Physician Practice business segment. Our business is now known as Altera Digital Health.
Editor’s note: This is part two of a blog series in recognition of Patient Safety Awareness Week.
Patient safety is always top of mind at Allscripts, and during Patient Safety Awareness Week, we take advantage of this excellent opportunity to increase awareness and recognize the work already being done to maximize patient safety. Patient safety is an ongoing, evolving process and at Allscripts, we remain committed to helping others maintain the highest level of patient safety measures, across all venues of care.
Let’s take a closer look at clinical risk identification and analysis. As noted in the figure below, clinical risk identification and analysis are the initial components of the overall clinical risk assessment process. This stage is crucial in capturing and understanding the nature of potential safety hazards. It is the foundation of the overall approach Allscripts takes to managing clinical risk and improving patient safety across all our client sites—including the safe use of our healthcare IT products and services.
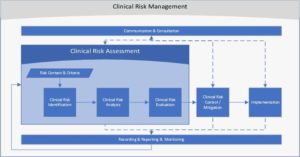
Figure 1: Allscripts Clinical Risk Management Process Flow
Risk identification focuses on potential safety hazards found in the field (by clients, partners or support analysts) and on a proactive assessment of new release content. The intent is to “cast a wide net” to identify potential risk factors, while ensuring the efficiency and effectiveness of follow-on analyses and evaluation processes. In doing so, we can properly assess the potential risk against established risk context and criteria. When this comes together, clinicians gain deeper insight into the risks, and more important, how to avoid them.
Risk analysis focuses on risk sources, consequences, events, and potential scenarios or use cases, and typically considers factors such as the likelihood of events or consequences, the nature and magnitude of consequences, overall complexity and connectivity, and the effectiveness of existing controls. Techniques used in risk analysis can be qualitative, quantitative or a combination of both. For example, to properly assess clinical risk of any safety events regarding medications, it is important to consider not only the specific workflow and specific medication, but also the potential impacts that can occur if the hazard were to occur with other medications and using alternative workflows.
As we move through 2021, watch for more blogs in this series, each highlighting a specific part of the patient safety workflow process.